From concept to market,
"Our technology enhances competitiveness"
About us






Our mission is to provide the medical industry with a comprehensive solution for the design, development, testing, manufacturing, and logistics of medical devices.
We have a Quality Management and Continuous Improvement System, supported by highly qualified personnel, enabling us to meet manufacturing best practices and the requirements of our clients and the regulatory standards governing our industry.
Vertical Integration
Your all-around answer to success
Startups
Turning visionary ideas into vibrant realities
Location
Boosting your margins with strategic proximity and efficiency
Services

Research and Development
Creating advanced, reliable, and effective medical devices ready to manufacture. Our R&D area is the root of the entire integrated process.

Contract Manufacturing
Producing high-quality medical devices with the capability of scalable production processes.
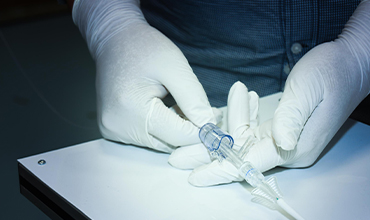
Quality Management
Rigorous testing and continuous improvement. Our commitment ensures the safety, effectiveness, and reliability of our products.

Sterilization
ETO sterilization provides a thorough and reliable method for ensuring the safety and sterility of the medical devices.

Validations
Strict evaluations of our equipment, process, sterilization and final product to ensure performance and reliability on every stage.

Product Certification
We run testing, provide documentation, and contribute in regulatory submissions to achieve certifications from global health authorities.

Product Certification
Approval from regulatory authorities to ensure compliance...

Contract Manufacturing
State-of-the-art facilities for the manufacturing of medical devices...
Certifications

ISO 13485: Medical Devices QMS
Enables us as manufacturers of medical devices and related services to increase our access to international markets.

FDA Registration
Commitment to compliance with regulatory standards for medical devices in the United States.

COFEPRIS NOM-241 Medical Devices GMP
Certified by the Government of Mexico as an organization that ensures good manufacturing practices for medical devices.
Frequently Asked Questions
Why Mexico?
Manufacturing medical devices in Mexico offers several key advantages, including cost-efficiency, proximity to major markets like the U.S., skilled workforce and beneficial trade agreements like USMCA.
Why us?
Choosing us means partnering with a reliable, quality-driven manufacturer that understands the unique advantages of producing medical devices in Mexico, ensuring your products are safe, effective, and market-ready.
How can I hire your services?
Contact us by leaving your contact information to schedule an initial meeting. We'll discuss your needs and provide a tailored proposal.
Can I schedule a visit to your facilities?
Yes, we welcome visits to our facilities! Please contact us in advance to schedule a tour so we can ensure a comprehensive and informative experience tailored to your interests.
Do you have FDA registration?
Yes, we are registered with the FDA and comply with all relevant regulations and standards.
News

BMC Secures FDA 510(k) Clearance
A US-based R&D company approached BMC Medical Manufacturing seeking a contract manufacturer for its "finished" product design.

Nearshoring: A prime opportunity
Monterrey is leveraging nearshoring to optimize benefits to its’ customers.

Why invest in Mexico?
Mexico's medical device industry has gained impressive momentum.
Advanced design tools, cutting-edge manufacturing technologies, and stringent quality systems to ensure our products meet high standards.
Clients




Our clients
We have been partnering with BMC Medical Manufacturing for several years, and their team of skilled engineers consistently impresses us with their technical expertise and attention to detail. They have an exceptional ability to turn complex ideas into practical solutions, ensuring that our products meet the highest quality standards. Their commitment to innovation and excellence has made a significant impact on our product development process.

Sintra Medical Team
Our experience with BMC Medical Manufacturing has been outstanding from start to finish. Their skilled engineers work seamlessly with our team, bringing a wealth of knowledge and innovative thinking to every project. The synergy between their R&D and manufacturing teams ensures that our devices are not only designed for optimal performance but also manufactured with precision and efficiency. Their commitment to quality and customer satisfaction makes them an invaluable partner in our product development journey.

Uphill Medical Team
We couldn’t be happier with the manufacturing services provided by this company. Their state-of-the-art facilities, combined with a meticulous approach to quality control, ensure that every device is produced to the highest standards. The team’s deep understanding of regulatory requirements and commitment to continuous improvement gives us confidence that our products are not only safe but also reliable. We are confident that their exceptional manufacturing capabilities will allow us to scale efficiently and meet the market demand with ease.

Basis Medical Team
Our Team

ALBERTO PEÑA
General Manager

OSMEL PÉREZ
Operations Leader

MARIAN RODRÍGUEZ
R&D Leader

ESTRELLA ROMÁN
Health Manager / Quality Supervisor

ANA LUCÍA GONZÁLEZ
Validation Supervisor

ADELAIDA MORENO
Production Supervisor

ANA YANCY PADRÓN
Accounting Administrator

SAMANTHA AGUILAR
Human Resources Generalist

ROBERTO HERNÁNDEZ
Maintenance Engineer

CARLOS ÁLVAREZ
Technical and Regulatory Consultant

MARTIN TYLER
Business Development

MITZI AVENDAÑO
Regulatory Affairs Responsible
Contact
Advanced design tools, cutting-edge manufacturing technologies, and stringent quality systems to ensure our products meet high standards.
USA Location: 2916 N Miami Ave. Ste 621. Miami FL 33127, USA.
Phone: +1 (786) 668 7715
Mex Location: Blvd TLC, Parque Industrial Millenium Apodaca, N.L. Mexico.
Mail: info@bmcmm.com
Hours: Mon - Fri de 9:00 a 18:00 hrs. UTC-06:00



